Gallery
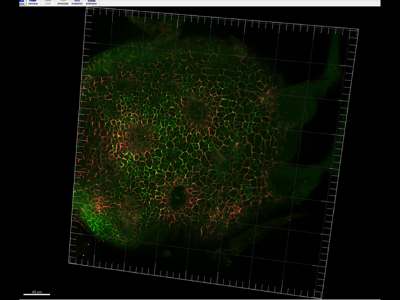
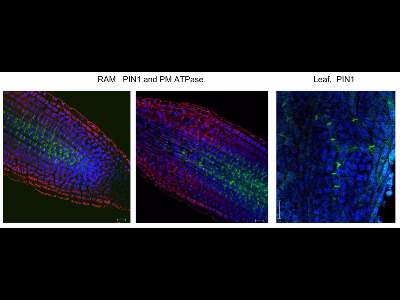
Immunolocalization in Arabidopsis leaf
PIN1 and PIN4 protein immuno localization in the 4 days old Arabidopsis leaf
Anti-PIN1 mouse monoclonal primary antibody (clone 10A7) diluted 1: 50 plus Alexa Fluor® 488 goat anti-mouse IgG as secondary antibody diluted 1: 800 (shown in green color) and anti-PIN4 rabbit primary antibody plus Alexa Fluor® 555 goat anti-rabbit IgG as secondary antibody diluted 1: 800 (shown in red color) were used.
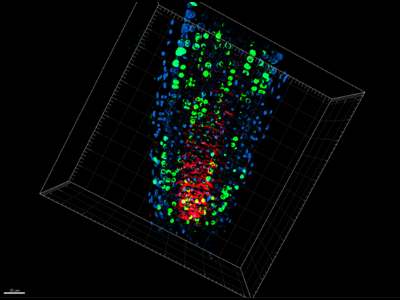
Detection of S-phase abundance and auxin transport protein in Arabidopsis roots
4 days old Arabidospis seedlings were incubated for 60 minutes with BrDU. Plants have been fixed and immunolocalization have been performed according to stadrad protocol. S-phase positive cells (BrDU) shown in green; co-localization with PIN1 in red color. 3D reconstruction was done with IMARIS.
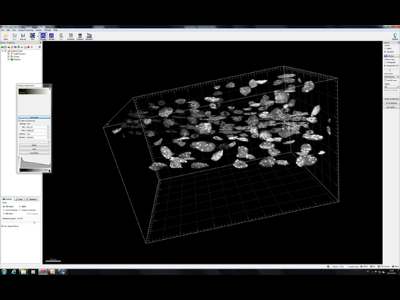
Cytoskeleton localization
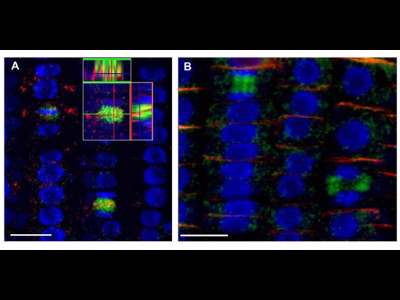 3D reconstruction Arabidopsis root epidermis cells undergoing telophase: co-localization of ß-Tubulin (TUB) and PIN1 in division plates.
3D reconstruction Arabidopsis root epidermis cells undergoing telophase: co-localization of ß-Tubulin (TUB) and PIN1 in division plates.
4 days old Arabidopsis seedlings were fixed for 30 min in formaldehyde. Panel A. Anti-PIN1 mouse monoclonal primary antibody (10A7) diluted 1:50 plus ALEXA Fluor ® 555 anti-mouse as secondary antibody diluted 1:800 (shown in green color) and anti-TUB (AS10 681) rabbit primary antibody diluted 1: 600 plus Goat anti-rabbit IgG (H&L), DyLight® 488 Conjugate (AS09 633) diluted in 1: 3000 as secondary antibody (shown in red color) were used. Panel B. Anti-PIN2 anti-guinea pig polyclonal primary antibody (clone A193) dilution 1:800 plus ALEXA Fluor ® 555 anti-quinea pig as the secondary antibody dilution 1:800 (show in green color) and anti-TUB (Agrisera, catalog number AS10 681) anti-rabbit primary antibody diluted 1: 600 plus Goat anti-rabbit IgG (H&L), DyLight® 488 Conjugate (AS09 633) as secondary antibody diluted in 1: 3000 (shown in red color) were used. Co-staining with DAPI visualized nucleus (shown as artificial color in blue).The Insertion in panel A shows an ortho-view of dividing cells.
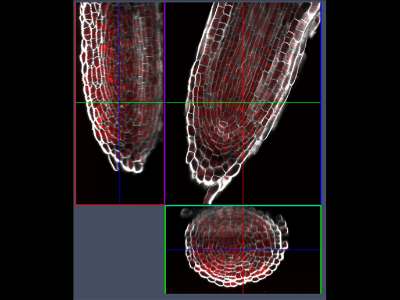
3D reconstruction of the elongation zone of the Arabidopsis roots
Protein immunolocalization in the Medicago sativa
 Medicago plants have been fixed, protein immunolocalization was done according to standard procedure. Plants have been co-staining with DAPI. PIN1 shown in green; ATPase in red and DAPI in blue. Scale bar: 20 µm.
Medicago plants have been fixed, protein immunolocalization was done according to standard procedure. Plants have been co-staining with DAPI. PIN1 shown in green; ATPase in red and DAPI in blue. Scale bar: 20 µm.
3D reconstruction of Medicago roots after calcofluor white and propidium iodine labeling.
Medicago sativa roots have been fixed and stained with propidium iodine (nucleus) and calcofluor white (cellulose/cell wall). Ortho projection have been shown.
Protein immunolocalization in Lycopersicum esculentum roots.
3 days old wheat seedlings were fixed for 30 min in formaldehyde. Anti-PIN1 mouse monoclonal primary antibody (clone 10A7) diluted 1: 50 plus Alexa Fluor® 488 goat anti-mouse IgG as secondary antibody diluted 1: 800 (shown in green color) and H+-ATPase (AS07 260) rabbit primary antibody plus Alexa Fluor® 555 goat anti-rabbit IgG as secondary antibody diluted 1: 800 (shown in red color) were used. Co-staining with DAPI visualize nuclei (shown as artificial color in blue). Scale bar: 20 µm. White arrow show PIN1 polarity.
A - PIN1 and ATPase in the stele; B - ATPase in the outer cell file. No PIN1 have been detected in these cells. Co-staining with DAPI visualized nucleus (shown as artificial color in blue).
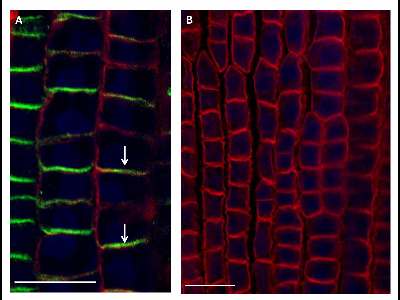
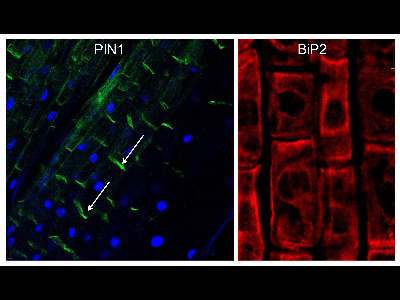
Protein immunolocalization in wheat roots.
3 days old wheat seedlings were fixed for 30 min in formaldehyde. Anti-PIN1 mouse monoclonal primary antibody (clone 10A7) diluted 1: 50 plus Alexa Fluor® 488 goat anti-mouse IgG as secondary antibody diluted 1: 800 (shown in green color) and anti-BIP2 (AS09 615) rabbit primary antibody plus Goat anti-rabbit IgG DyLight® 549 Conjugate (AS09 642) as secondary antibody diluted 1: 3000 (shown in red color) were used. Co-staining with DAPI visualize nuclei (shown as artificial color in blue). Arrow demonstrated polar PIN1 localization.
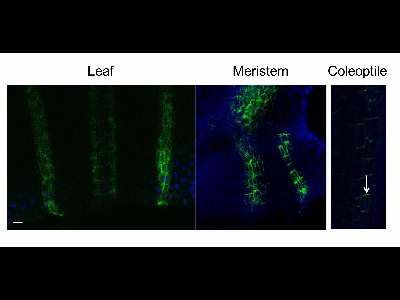
Protein immunolocalization in above-ground cereals organs.
3 days old wheat seedlings were fixed for 30 min in formaldehyde. Anti-PIN1 mouse monoclonal primary antibody (clone 10A7) diluted 1: 50 plus Alexa Fluor® 488 goat anti-mouse IgG as secondary antibody diluted 1:800 (shown in green color) were used. Co-staining with DAPI visualize nuclei (shown as artificial color in blue). Arrow demonstrated polar PIN1 localization. Scale bar: 20 µm.