Plant growth regulation in tissue culture in vitro
There are 2 types of in vitro tissue culture options:
-
Clonal propagation, which widely has been used in agriculture for propagation of plants like flowers, some specific genotypes etc. This option have beem used for the plant propagation through activation of the axillary buds which were formed in majority of dicotyledon stem-like plants during growth (Solanacea, Fabeaceea species). Axillary bud represent portion of non-differentiated dormant cells that do not requireadditional external hormonal signal. However, addition of low cytokinin concentration (100-200 nM) may have a positive effect on activation of axillary buds activation, probably through activation of internal auxin biosynthesis. However, method of clonal propagation is hardly acceptable for non-stem plants like rosette Brassicaceae or majority of cereals (wheat, barley, rye etc). These plants formed only one meristem during vegetative growth. Stem formation occurred only after transition to generative stage. However, cereal plants also can be propagating through intercalary meristem during generative growth, but with many precautions. In the case of Brassicacea plants one can use propagation of the flowering buds. In our hand, we sucessfully propagated Arabidopsis plants and can got up to 10 independent stem from 1 plant.
In addition to growth regulators, majority of the medium for clonal propagaqtion in vitro include 2-3% sugar. Presence of sugar is extermely essentail for clonal propagation because sugar is involvemnt ion the regulation of the auxin response at multiply level (Pasternak et al., unpublished). Similarly, presence of enough intensive light is also crucial, because light is require not only for sugar production, but alos for photomorphogenesis, ea, creation of the new sites of auxin production in de novo formed leaf. In many cases we observed insufficient illumination in plant growth chamber (40-70 µmol/sq.m/sec what can be achieved by lamp in many case and which is not enough for efficient propagation). We must mention that natural sun light have 200-250 µmol/sq.m/sec. The presence of sugar even more important for activation of axillary buds, because sugar is an important part of the hormonal (auxin) signaling and can rescue low photosynthesis ratio in the absence of differentiated photosynthetic leaves. Sucrose is a most widely accepted sugar source, however, glucose also can be used. One should consider that fact that glucose can not be autoclaved (during autoclaving it cobnvert to 2 milk acid molecules), but shoudl be sterlized by filtering.
Growth of axillary buds require internal auxin biosynthesis and its polar transport. These processes require coordination of TAA1/YUCCA auxin biosynthesis pathway with expression of auxin transport efflux protein (PIN1). Axillary buds in planta already contains group of the non-differentiated cells with already active PIN1 protein and YUCCA mRNA. So, upon removing from the apical dominance, these cells were able to rapid induce auxin synthesis, its polar transport and, therefore, buds start to develop rapidly.
The next step in the clonal propagation is a rooting of the buds. The first step in the rooting process is an induction de novo of root apical mersitem. This step require exogenosu auxin and sugar: buds-derived or form the culture medium. In most cases sugar concentartion should not excees 1-2%, higher concenhtartion will have inhibitory effect. Moreover, for the majority of plant species concentration of exogenous auxin should alos relatively low: higher auxin concentartion may induce callus formation instead of roots.
-
De novo induction of meristemic cells from differentiated one represent second approach in tissue culture in vitro, including plant regenaration from explants like leaf, stem, roots, as well as isolated protoplasts. These approaches suitable for polant transfroamtion, somatic hybridization etc.
These approaches require total cells reporgramming, what can be achieve only by applications of external phytohormones at relatively high concentration because intialy differentited cell does not have active hormone biosynthesis.
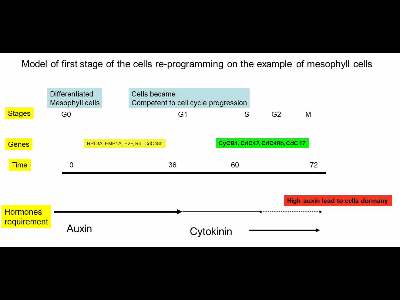
Generally, cells reprogrammimg include several different steps what have quite different requirements to the external hormones. Briefly, such requirement can be summarized in the following model:
The first stage of cell re-programming from differentiated somatic cells include transition from differentiated cells (G0) to cycling one (G1). This step require application of the high auxin dosage for short period (10-48 hours). Duration of the treatments were largely dependent from the intial explants stages. If one have used fully differentiated mesophylll cells, such duration can be very long with high auxin dose (Medicago leaf protoplasts, for example, require up to 10 mkM 2,4-D). However, for some other plant species with lower level of differentiation even 2 mkM NAA for short time will be enough for G0-G1 transition (Nicotiana),
One can determine level of cells differentiation by determination of the chromatin and nucleolus structure in explants.
However, exact concentarions of hormones were largely dependent from explants, and even cells types in explant whcih are in different status. As example we can look on typical explant: leaf of dycotelon plants like Arabidopsis. Fully expanding leaf contain mesophyll cells, which is fully differentitated, dies not have auxin biosynthesis and transport activities. However, there rae also a number of other cells tyoes, including bundle sheath cells. These cells demonstrated relatively high level of auxin accumilation, capacity to auxin biosynthesis through Yucca pathway and auxin transport capacity be presence of auxin transportes like PIN1 on mRNA and protein level.
Different plant species have different level of diierrentiation in the mesophyll cells. Thats why ability to re-enter back cell cycle between different species, and even between different leaf in the same plant also highly variable. As a marker of cell differentiation one can use chromatin structure and nucleolus activity. These parametrs can be easy determined by double staing with DAPI and propidium iodine. Howevre, one should consider that in this case the best way is to have as a control nucleus form the cycling cells (meristemic) for comparison.
The other serious problem with cultivation of the mesophyll cells is ploidy level. Unapproppriate nutrient balance in the medium for donor plants may lead to distrubance in intrenal hormonal balance during leaf growth. In turn, this will lead to high level of DNA reduplications. Such high ploidy level has been observed in Arabidopsis plants growing on MS medium (ref).
Cereal (monocotyledons) may represent special kind of plants with specail requirements for cultivation in vitro. Namely, majority of cereal species like wheat, barley etc. demonstrtade very rapid cell differentiation upon exit cell cycle in planta. This is very clear demonstrated on the examoles of barley leaf: only 2-4 segment closed to meristem were capable to re-neter backl to cell cycyle, and give rise to new plants. The more distal part of leaf were uncappable to re-enter cell cycle even with very high auxin concentrations, or can oass only restricted number cell divsion and go to necrosis. Tha's why for these species only immature explants (embryos, influoresences) were capable to formed highly embryogenic callus. Namely, scutelluar tissue from 12-14 days old wheat embryos after tretamemt with exogenous auxin (2,4-D) capable to fo9rmed friable embryohenic callus. At the same time, similar callus can not be obtained from embryos itself because cells of the embryos alredy pass specifcication step and undergo "normal" developmental programm even in the presence of high concentarion of external auxin. As a conclusion, only optimal explants will lead to sucess in cereal tissue culture and totipotence can not be induced by changes in exteranl hormones or/and medium components. In the case of differentiated tissue even high concentartion of strongest external auxin not capable to induce callus formation from mature tissue.
Preparation of the plant tissue culture medium.
Composition and preparation of the medium for plant cultivation have a primary importance for sucessful cultivation.
Plant nutrition is a key factor in determination of plant metabolism and architecture. Any disturbance in plant nutrition (nutritional stress) may lead to significantly alteration in plant metabolism and alteration in plant shape. If wild type plants have significant possibilities for adaptation to the nutritional stress, majority of the mutants have reduced such possibilities. That’s why, when one design mutant characterization they must be ensure to avoid nutritional stress. Last decade many investigations have be done on Arabidopsis plants, including mutants characterization and investigation of the gene expression in details. However, for unknown yet reason, majority of the researches used for the purpose as a control high nutritional stress conditions (MS medium).
There are 6 macronutrients plants can get from the culture medium. Among them Nitrogen, Potassium and Phosphorous are the primary macronutrients, what require in a high quantity. The other 3 (Calcium, Sulfur and Magnesium) require in a lesser quality. However, plants do not require high amount of chloride, it is either neutral, or rather highly toxic for couple of plants (Eaton F.M., 1942), like tomato, potato, and some Arabidopsis mutants.
There are several typical pitfalls during preparation of the plant tissue culture medium:
- Precipitation of the salt. It is long times well known that majority of plant tissue culture medium have a high tendency to form precipitation through time because the absence of organic buffers. Interesting, that precipitation did not formed immediately after salt mixing, but occurred through time (after 2 days), and only in the presence of all components together: macro salt, Fe-chelat and micro salt separately did not form any precipitate. The first attempt to find nature of precipitation was performed by Dalton et al: “Dalton C. C., Iqbal K. and Turner D. A. (1983) Iron phosphate precipitation in Murashige and Skoog media. - Physiol. Plant. 57: 472-476.” Several authors suggest different possibilities to avoid precipitation “Schenk N., Hsiao K. and Bornman C. (1991) Avoidance of precipitation and carbohydrate breakdown in autoclaved plant tissue culture media Plant Cell Reports Volume 10, Issue 3, pp 115-119”. However, according to our experience, simple addition of even 100 mg/l casein hydrolysate (Bacto-Tryptone) very effectively preventing precipitation and serve as physiological buffer. Interesting, that there are 2 commercially available MS salt (Sigma M5524) and Duchefa (M0241) have a different composition (Duchefa require 100 mg/l more fresh weight of the powder) and different ability for precipitation (M5524 formed precipitate very rapid).
- pH stabilization. As we mention before, majority of plant tissue culture mediums does not have physiological buffering capacity. In addition, autoclaving itself may significantly alter medium pH. That’s why many people use 5 and even 10 mM MES in the medium to prevent pH changes. Even more, some people suggested to use Na-MES instead of MES, which itself serve as high stress factor (5 or 10 mM Na caused slat stress). Moreover, researches should also take into account that 5 mM MES require additional 5 mM KOH, so, K+ concentration were changes significantly. Moreover, MES also contain sulfur, which, under certain conditions, may have a significant role itself. For example, Xin Yu reported that 10 mM MES almost completely abolish effect of BSO or GSH deficient mutant (??). It is not clear so far did MES effect related with sulfur metabolism, or with additional K in the medium. At the same time, casein alos serve as a good buffer, at least during medium preparation. In many case addition of 100 mg/l of the casein was stabilized pH of the medium on the usual level (5.8) and no adjustment required any more. As conclusion, MES shiuld be used with high precaution, others effects of the MES must be consider. In addition, in some cases MES have a very negative role on plant growth because plants require pH changes during growth. For example, Medicago cell culture (both suspension cells and leaf pp, Pasternak et al., 2002) lead to strong medium acidifications. However, addition of the MES has an extremely negative effect of cell development. As conclusion, pH stabilization may have a very positive effect on development (at pH5 Arabidopsis roots almost stop growth and demonstrated strong abnormalities) in some case, but may have a negative effect in some species. Moreover, MES itself may interact with plants development, especially with sulfur or GSH -deficient ones.
- pH during the culture. Different plants species have different optimal soil pH during growth in the field and this differences seems to be correlated with plant behavior in vitro. For example, among cereal species rice is one of the most suitable for embryogenesis/plant regeneration. Interestingly, that optimal pH for plant growth in soil is 4-4.5, with lower level for Japonica rice. And this fact correlated well with plant behavior in vitro. From the other side, other cereal – barley- has a very high soil pH. In parallel, barley is one of the most difficult species for culture in vitro. Interesting, we do not able to stabilize pH of the standard medium for barley even with 20 mM MES due to very high proton pumping activity – pH of the medium in the presence of explant were dropped to 4 after 24 hours in the culture.
Plant medium composition.
Composition of the plant medium is crucial for sueccesfull plant growth and regeneration. Paradoxically, commonly accpetred MS (Murashige and Skoog) medium is not suitablke for majority of plant species. Originally this medium has been design for the tobacco callus, contains very high amount of Nitrogen, very low phosphates, very high Zn and very low Cu. So, N:P:K ratio in MS medium is 60:20:1.25 and Zn/Cu ratio is 1200:1. Cu is essentail co-factor of numerous enzymes, including Cu/Zn superoxide dismutase, what sereve as an important ROS scavenger. Cu deficency in MS medium lead to problem with ROS scavenger as well. Moreover, for unknown reason MS medium contain very high concentration of chloride (4 mM). however, chloride is not essentailelement for plant development, espesially in such high concebtrations. Alltogether, these nutrients balance are too far from optimum for plant development, however can be suitable for non-organized plant growth like callus induction. Many plant species during cultivation on MS medium demonstarted symptoms of stress reaction. That's why a numbers of Arabidopsis mutants demonstrated severe defects on MS medium, but have been recovered on more suitbale one. Moreover, one should also consider the fact that different stage of plant development have a different nutrient requirement. Namely, for vegetative growth majority of the plants require high nitrogen contentens to accumulate high amount of biomass. However, during and fater transition to generative growth (flowering, seeds formation) high nitrogen have an inhibitory effect. Similar picture have been observed during plant regeneartion formthe tissue culture: callus induction require high nitrogen, and can be acivied even with low phospate and copper. However, for any morhogenesis, including somatic embryo and axiallary buds induction from bundle sheath cells high phosphate and copper may require.
In addition, different plant species have a completely different nutrient requirements. So, in ideal case, instead of alteration in hotmonal balance during culture in vitro, one should use different nutriemt composition for keeping more suitable internal hormonal balance in tissue, The high exteranl hormone level, in combination with non-optimal nutrient balance may lead to numerous problems,like antocyanin production and vitrification.
Vitrification.
Vitrification is a probelm in tissue culture in vitro, mainly during de novo plant regeneration or plant micropropagation. Vitrification is much more appeared on the medium solidified with Gerlite or Phytagel. Despite it was mentioned that geröite or phytageö may serve as agar substituion, both compounds is never solidified in the absence of divalent cations (Ca, Mg etc). It means that the "real" concentartion of the divalent cations on the medium with phytagel were significantly lower to compare with the agar-solidified medium. So, one of the strategy to avoid vitrification is to avoid gerlite/phytagel usage for plant regeneration and micropropagation.
Somaclonal variation.
Somaclonal variation can be difine as high genetic variability in the plant which originated form somatic cells. Somaclonal variation appeared only if somatic plant cells pass through long peroid of un-organized growth (callus). The main origina of such variation is high genome instability due to relatively high level of the reactive oxygen species in the callus (un-organized cells). In the case of direct induction of axillary buds from competent bundle sheath cells somaclonal variation is not the case because the absence of the stage of un-organized growth.
Cell Suspension culture
Cell suspension culture can serve as an ideal tool for investigation of the several biological process like cell cycle investigation. There are 4 classical cell suspension lines: tobacco BY2 line, Medicago A2 line, Arabidopsis MM2 line and maize B73 line. All on these lines were maintenined as uniform cells population and, except B73, and partially Arabidopsis MM2 line, loose ability to regenerate plants. Moreover, our numerous attempt to establish homogenous suspension culture from the other lines, including Arabidopsis and Medicago were unsuceefull. In all of case cells have a high tendency to aggregation (growth as a big clasters) and tendency to induction of the morphogenesis. Why this is the case? Cell division require internal hormones (auxin and cytokinin) synthesis, expternal hormones have only a pulse effect. In plants auxin biosyntehsis occurred through TAA1_Yucca pathway, which strictly associated with dormant non-dividing cells (in Arabidpsois RAM it is QC and intials). That's why wild type cells can not exist as a continues single cells populations. We have found that existing cells suspension nemaly Medicago A2, have an additional auxin biosyntehsis pathway through IAAH amd IAAM genes. In the whole plant level these genes expressed only in the meristemic zone (dividing cells), but once after induction of cell suspension, they became expressed in all cells. Expression of these genes is the main reason why A2 cells loose totipotence.