Research
Contents
- Arabidopsis root as a model of the interaction between auxin metabolism and cell cycle
- Redox
- Dynamics of Plant Stem Cells and their Niches
- Lateral root development: an emerging story
- Plant Cell Technology
Part I. Arabidopsis root as a model of the interaction between auxin metabolism and cell cycle
Introduction
The investigation of the link between auxin distribution and cell cycle progression is an important for our understanding of the mechanism of root growth and development. Given the fact that even in the simple root like Arabidopsis each cell continuity have a different fate, different auxin level and direction of auxin transport. In turn, this lead to different cell cycle regulation in the term of cell cycle exit towards differentiation. Moreover, even simple Arabidopsis root has a multiply mode of symmetry: radial in the trichoblast, cortex and endodermis and bilateral in the rest cell files. In order to achieve a detailed functional and quantitative understanding of how root cells acquire their fates and communicate with each other their development must be studied in the three-dimensional (3D) context of the whole organ. We recently developed an intrinsic root coordinate system (iRoCS) which enabled the direct quantitative comparison between root tips at single cell resolution.
Publications
The iRoCS Toolbox – 3D Analysis of the Plant Root Apical Meristem at Cellular Resolution
Authors: Thorsten Schmidt, Taras Pasternak, Kun Liu, Thomas Blein, Dorothée Aubry, Alexander Dovzhenko, Jasmin Duerr, William Teale, Franck A. Ditengou, Hans Burkhardt, Olaf Ronneberger, Klaus Palme Plant J., accepted (2014)
Summary To achieve a detailed understanding of processes in biological systems, cellular features must be quantified in the three-dimensional (3D) context of cells and organs. We introduce the intrinsic Root Coordinate System (iRoCS) as a reference model for the root apical meristem (RAM) of plants. iRoCS enables direct and quantitative comparison between the root tips of plant populations at single cell resolution. The iRoCS Toolbox automatically fits standardised coordinates to raw 3D image data. It detects nuclei or segments cells, automatically fits the coordinate system, and groups the nuclei/cells into the root’s tissue layers. The division status of each nucleus can also be determined. The only manual step required is to mark the quiescent centre, the origin of iRoCS. All intermediate outputs can be refined if necessary. The ability to learn the visual appearance of nuclei by example allows the iRoCS Toolbox to be easily adapted to different phenotypes. We provide the iRoCS Toolbox as open source software package, licensed under the GNU General Public license (GPL) to make it accessible to a broad community. To demonstrate the power of the technique, we measured subtle changes in cell division patterns caused by modified auxin flux within the Arabidopsis thaliana RAM.
Usage of scalar and vectorial grayscale based invariant features for localization and classification of cell cycle events in Arabidopsis RAM
Abstracts: P07-051 FESPB 2008, Tampere, Finland
Authors: T. Pasternak, T. Schmidt, J. Schulz, O. Ronneberger, H. Burkhardt, A. Dovzhenko and K. Palme
Summary Details of cell cycle regulation are being uncovered in the case of model systems like suspension cells, but less progress are made in unraveling how these molecular events regulate growth processes at the whole organism level. The main problem is the absence of a set of analytical tools that are powerful enough to determine cell cycle events in whole organisms. Appropriate methodology has been pioneered in the last century and is now defined as ‘kinematic analysis’. This analysis requires an accurate and predictive model of the distribution of cell cycle events through the whole plant (in 4-D) and generation of mathematical models which will allow to predict and describe cell behavior in each cell fi le, including both generation of new cells, differentiation and cell maturation. Here we developed a 4-D model, which shown dynamics of S and M-phase of cell cycle. Detailed analysis of the architecture of the RAM revealed a number of important observations which were integrated in the model development. We show that the mitotic index of the epidermal cell file is significantly lower in comparison with other cell files. The data analysis showed a significant variation of nucleolus volume among different cell files in the RAM, thus we propose to use the size of the nucleolus as a novel marker to distinguish different cell files. The proposed model allows to correctly quantifying cell cycle events in the RAM and demonstrates changes in nucleolus sizes during cell maturation.
Part II. Redox
Introduction
Redox is an important mirror of cell stage and cell fate in plants. We have studied how low molecular weight antioxidants and its redox level affected plant development at plants, organ and cellular level.
Publications
Dehydroascorbate and glutathione regulate the cellular development of Nicotiana tabacum L. SR-1 protoplasts.
Authors: Potters, G., Jansen, M. A., Horemans, N., Guisez, Y., & Pasternak, T. (2010). In Vitro Cellular & Developmental Biology-Plant, 46(3), 289-297.
Summary A Nicotiana tabacum L. SR-1 leaf protoplast system was used to study the effects of dehydroascorbate and glutathione on cellular development. We found that dehydroascorbate is readily taken up by protoplasts and internally reduced to ascorbate. Concomitantly, cell division was inhibited and cell expansion stimulated. In this respect, dehydroascorbate counteracted auxin-mediated leaf protoplast development. In contrast to the effects of dehydroascorbate, glutathione-induced cell dedifferentiation, and this effect is similar to that mediated by high auxin concentrations. We conclude that dehydroascorbate and glutathione affect the auxin-mediated regulation of cellular development. Therefore, the biological role of these compounds extends beyond stress tolerance and defense.
The thiol compounds glutathione and homoglutathione differentially affect cell development in alfalfa (Medicago sativa L.)
Authors: T Pasternak, H Asard, G Potters, MAK Jansen Plant Physiology and Biochemistry, 2014
Summary Glutathione (GSH) is an important scavenger of Reactive Oxygen Species (ROS), precursor of metal chelating phytochelatins, xenobiotic defence compound and regulator of cell proliferation. Homoglutathione (hGSH) is a GSH homologue that is present in several taxa in the family of Fabaceae. It is thought that hGSH performs many of the stress-defence roles typically ascribed to GSH, yet little is known about the potential involvement of hGSH in controlling cell proliferation. Here we show that hGSH/GSH ratios vary across organs and cells and that these changes in hGSH/GSH ratio occur during dedifferentiation and/or cell cycle activation events. The use of a GSH/hGSH biosynthesis inhibitor resulted in impaired cytokinesis in isolated protoplasts, showing the critical importance of these thiol-compounds for cell division. However, exposure of isolated protoplasts to exogenous GSH accelerated cytokinesis, while exogenous hGSH was found to inhibit the same process. We conclude that GSH and hGSH have distinct functional roles in cell cycle regulation in Medicago sativa L. GSH is associated with meristemic cells, and promotes cell cycle activation and induction of somatic embryogenesis, while hGSH is associated with differentiated cells and embryo proliferation.
Plastid-Localized Glutathione Reductase2–Regulated Glutathione Redox Status Is Essential for Arabidopsis Root Apical Meristem Maintenance
Authors: X Yu, T Pasternak, M Eiblmeier, F Ditengou… The Plant Cell Online, 2013
Summary Glutathione is involved in thiol redox signaling and acts as a major redox buffer against reactive oxygen species, helping to maintain a reducing environment in vivo. Glutathione reductase (GR) catalyzes the reduction of glutathione disulfide (GSSG) into reduced glutathione (GSH). The Arabidopsis thaliana genome encodes two GRs: GR1 and GR2. Whereas the cytosolic/peroxisomal GR1 is not crucial for plant development, we show here that the plastid-localized GR2 is essential for root growth and root apical meristem (RAM) maintenance. We identify a GR2 mutant, miao, that displays strong inhibition of root growth and severe defects in the RAM, with GR activity being reduced to ∼50%. miao accumulates high levels of GSSG and exhibits increased glutathione oxidation. The exogenous application of GSH or the thiol-reducing agent DTT can rescue the root phenotype of miao, demonstrating that the RAM defects in miao are triggered by glutathione oxidation. Our in silico analysis of public microarray data shows that auxin and glutathione redox signaling generally act independently at the transcriptional level. We propose that glutathione redox status is essential for RAM maintenance through both auxin/PLETHORA (PLT)-dependent and auxin/PLT-independent redox signaling pathways.
Part III. Dynamics of Plant Stem Cells and their Niches
Taras Pasternak, Edwin Groot
Summary After germination, cells that leave the meristem to become part of newly formed organs (leaves, internodes, floral organs, primary and lateral roots) are replenished by the stem cells. A communication network that includes hormones and signal peptides maintains the stem cells in a dedifferentiated and slowly cycling (longer G1 phase) state. In addition to their self-renewal capability, longer cell cycle and lack of differentiation, stem cells have a distinct chromatin state, as revealed by DNA and RNA-intercalating dyes and a distinct protein storage vacuole type. Interestingly, cultured single cells during induction of somatic embryogenesis also have features of stem cells, such as auxin accumulation, very small size, and extension of the cell cycle duration, protein storage vacuole abundance and nuclear morphology. Upon recovery from a quiescent stage these cells give rise directly to somatic embryos. One neglected aspect of stem cells is their dynamics. During the life cycle of a plant, a stem cell population can increase during induction of the inflorescence meristem. Stem cell niches form de novo from differentiated tissue during lateral root and nitrogen fixing nodule induction. Endogenous auxin accumulation plays a role in this process. Finally, stem cells decrease in number as a root ages and during termination of floral and inflorescence meristems. Genetic and exogenous perturbations, like biotic and abiotic stress can alter stem cell number in the root and shoot niches. Increases in stem cell number are often erroneously interpreted as a shortening of their cell cycle. Alternatively, both daughters enter a quiescent state at the G1 phase, rather than one of the daughters entering the meristem and continuing to cycle. Live imaging with appropriate markers avoids these misinterpretations. Because stem cells are a small fraction of the meristem, the behaviour of stem cells is diluted by other tissues when using the whole-organ techniques of Western blots and transcriptomic analyses. Thus, in situ techniques are more desirable to test hypotheses of stem cell function and for the studying of gene interactions.
Part IV. Lateral root development: an emerging story
Introduction
Root system architecture plays an important role in determining nutrient and water acquisition and is modulated by endogenous and environmental factors, resulting in considerable developmental plasticity. Architecture of the root system was regulated by environment, a phenomena known as SIMR (Pasternak et al., 2005; Potters et al., 2007). Lateral roots extend horizontally from the certain cells of the primary root and serve to anchor the plant securely into the soil. This branching of roots has a primary importance in nutrient uptake and hormone production.
Publications
3D analysis of mitosis distribution highlights the longitudinal zonation and diarch symmetry in proliferation activity of the Arabidopsis thaliana root meristem
Authors: Lavrekha, V. V., Pasternak, T., Ivanov, V. B., Palme, K., & Mironova, V. V. The Plant Journal, 92(5), 834-845. (2017)
Summary To date CYCB1;1 marker and cortex cell lengths have been conventionally used to determine the proliferation activity of the Arabidopsis root meristem. By creating a 3D map of mitosis distribution we showed that these markers overlooked that stele and endodermis save their potency to divide longer than the cortex and epidermis. Cessation of cell divisions is not a random process, so that mitotic activity within the endodermis and stele shows a diarch pattern. Mitotic activity of all root tissues peaked at the same distance from the quiescent center (QC); however, different tissues stopped dividing at different distances, with cells of the protophloem exiting the cell cycle first and the procambial cells being the last. The robust profile of mitotic activity in the root tip defines the longitudinal zonation in the meristem with the proliferation domain, where all cells are able to divide; and the transition domain, where the cell files cease to divide. 3D analysis of cytokinin deficient and cytokinin signaling mutants showed that their proliferation domain is similar to that of the wild type, but the transition domain is much longer. Our data suggest a strong inhibitory effect of cytokinin on anticlinal cell divisions in the stele.
A 3D digital atlas of the Nicotiana tabacum root tip and its use to investigate changes in the root apical meristem induced by the Agrobacterium 6b oncogene
Authors: Pasternak, T., Haser, T., Falk, T., Ronneberger, O., Palme, K., & Otten, L. The Plant Journal, 92(1), 31-42. (2017)
Summary Using the intrinsic Root Coordinate System (iRoCS) Toolbox, a digital atlas at cellular resolution has been constructed for Nicotiana tabacum roots. Mitotic cells and cells labeled for DNA replication with 5‐ethynyl‐2′‐deoxyuridine (EdU) were mapped. The results demonstrate that iRoCS analysis can be applied to roots that are thicker than those of Arabidopsis thaliana without histological sectioning. A three‐dimensional (3‐D) analysis of the root tip showed that tobacco roots undergo several irregular periclinal and tangential divisions. Irrespective of cell type, rapid cell elongation starts at the same distance from the quiescent center, however, boundaries between cell proliferation and transition domains are cell‐type specific. The data support the existence of a transition domain in tobacco roots. Cell endoreduplication starts in the transition domain and continues into the elongation zone. The tobacco root map was subsequently used to analyse root organization changes caused by the inducible expression of the Agrobacterium 6b oncogene. In tobacco roots that express the 6b gene, the root apical meristem was shorter and radial cell growth was reduced, but the mitotic and DNA replication indexes were not affected. The epidermis of 6b‐expressing roots produced less files and underwent abnormal periclinal divisions. The periclinal division leading to mature endodermis and cortex3 cell files was delayed. These findings define additional targets for future studies on the mode of action of the Agrobacterium 6b oncogene.
Part V. Plant Cell Technology
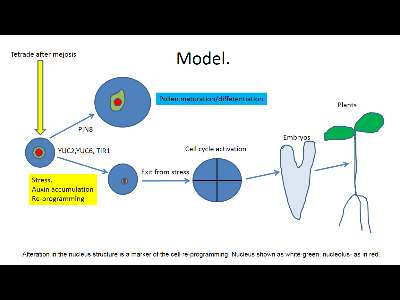
Pollen embryogenesis and its application in plant breeding and biotechnology.
The switch of gametophytic program to pollen embryogenesis with the further formation of the homozygous plants for production of new isogenic lines has a fundamental importance for plant breeding. This switch has been induced by stress like heat stress etc. However, molecular mechanism of such switch is unclear and no specific genes responsible for pollen embryogenesis have been detected so far.
Here we proposed hypothesis that switching of developmental program towards pollen embryogenesis is a part of general pathway of somatic cell re-programming and regulated epigenetically through regulation of the chromatin structure.
That’s why numerous attempts to find specific genes for pollen embryogenesis were unsuccessful.
Chromatin dynamics is a key epigenetic regulator of cell re-programming from differentiated somatic to dividing and especially to totipotent one. From this point of view transition of somatic cell to embryogenic (totipotent) stage can be dividing in 2 different steps (stages) which characterized by different chromatin dynamics. At the first stage cell which have a defined fate in planta (mesophyll cells, pollen etc.) should reactivate cell cycle. However, in planta cells which undergo rapid cell division already establishes cell fate and cannot be considering as totipotent without transition to de-differentiated stage. Correspondingly, only stem cells can be considered as totipotent. That’s why second step, ea. transition from dividing cell to totipotent represent a key step for realization of the totipotency program. The key differences between dividing and totipotent cells is a chromatin dynamics, which regulated rather epigenetically through HDAC activity and directly dependent from key plant phytohormone auxin. The fact that majority of the stress-responses in plants were regulated epigenetically fit well with the fact that stress is necessary for induction of totipotency as second step in cell re-programming (Chinnusamy, V., & Zhu, J. K.).
Pollen after meiosis is a relatively cell-cycle active cells which in planta undergo to maturation (partial differentiation) with further significant vacuolar growth (pollen tubes growth).
However, under certain experimental condition one can prevent further pollen differentiation and switch the program to cell cycle activity. This process is named “nuclear reprogramming” (Gurdon and Melton, 2008). “Nuclear reprogramming” can most simply be defined as a process of alteration in the nucleus structure (chromatin dynamics).
In the case of the pollen such switching and cell de-differentiation (“nuclear re-programming”) were accompanied by rapid changes in the chromatin structure (Testillano, P. S., et al., 2000). In addition, it has been recently shown such changes in nucleus organization during pollen embryogenesis were regulated by DNA methylation (El-Tantawy, A. A.et al., 2014).
Chromatin structure (nucleus shape, density, and even in more extension, nucleolus function) is an excellent marker of cell re-programming from somatic (pollen) to embryogenesis.
As it has been shown previously (Schmidt et al., 2014), cell in the de-differentiated stage in planta were characterized by extremely specific nucleus shape (round with very small size) and a very small nucleolus. These features can be easy determined by double DAPI/PI labeling (ref).
So far there is only one factor which is known to induce de-differentiation: auxin under high initial internal accumulation. It is also well-known that stress factors like high temperature (33° C), and even in more extension ASA and lactones, serve as an ideal factor to induced cell de-differentiation. The involvement of the auxin in pollen development have been pointed by high activity of the auxin biosynthesis genes YUC2 and YUC6 during pollen development, as well as activity of TIR1 and AFB1,2,3 at the stage after meiosis (Cecchetti, V. et al., 2008). The next possible factor what regulate pollen embryogenesis is auxin transporter PIN8. Loss of PIN8 function prevents pollen maturation and may led to switching of the pollen to hametophytic pathway. The involvement of the chromatin structure alteration in the pollen re-programming to embryogenic pathway has been confirmed in the recent investigation as well (Li. H et al., 2014).
Literature
Cheng Y.,Dai X., Zhao Y. (2006)
Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. 20, 1790–1799
Cecchetti, V., Altamura, M. M., Falasca, G., Costantino, P., & Cardarelli, M. (2008).
Auxin regulates Arabidopsis anther dehiscence, pollen maturation, and filament elongation.
The Plant Cell Online, 20(7), 1760-1774
Chinnusamy, V., & Zhu, J. K. (2009).
Epigenetic regulation of stress responses in plants.
Current opinion in plant biology, 12(2), 133-139
El-Tantawy, A. A., Solís, M. T., Risueño, M. C., & Testillano, P. S. (2014).
Changes in DNA methylation levels and nuclear distribution patterns after microspore reprogramming to embryogenesis in barley. Cytogenet Genome Res, 143, 200-208
Feher, A., Pasternak, T. P., & Dudits, D. (2003).
Transition of somatic plant cells to an embryogenic state.
Plant Cell, Tissue and Organ Culture, 74(3), 201-228
Gurdon J. B., Melton D. A. (2008).
Nuclear reprogramming in cells.
Science 322, 1811-1815
Li, H., Soriano, M., Cordewener, J., Muiño, J. M., Riksen, T., Fukuoka, H., ... & Boutilier, K. (2014).
The Histone Deacetylase Inhibitor Trichostatin A Promotes Totipotency in the Male Gametophyte.
The Plant Cell Online, tpc-113
Sakata, T., Oshino, T., Miura, S., Tomabechi, M., Tsunaga, Y., Higashitani, N., ... & Higashitani, A. (2010).
Auxin reverse plant male sterility caused by high temperatures.
Proceedings of the National Academy of Sciences, 107(19), 8569-8574
Schmidt, T., Pasternak, T., Liu, K., Blein, T., Aubry‐Hivet, D., Dovzhenko, A., ... & Palme, K. (2014).
The iRoCS Toolbox–3D Analysis of the Plant Root Apical Meristem at Cellular Resolution.
The Plant Journal. 77 (5), 806-814
Testillano, P. S., Coronado, M. J., Seguı́, J. M., Domenech, J., Gonzalez-Melendi, P., Raška, I., & Risueno, M. C. (2000).
Defined nuclear changes accompany the reprogramming of the microspore to embryogenesis.
Journal of structural biology, 129(2), 223-232